When we visit a national park or look at the skyline of a city, often we do not enjoy a clear vista -- a white or brown haze hangs in the air and affects the view. This haze is not natural. It is caused by man-made air pollution, often carried by the wind hundreds of miles from where it originated.
Typical visual range in the eastern U.S. is 15 to 30 miles, or about one-third of what it would be without manmade air pollution. In the West, the typical visual range is 60 to 90 miles, or about one-half of the visual range under natural conditions. Haze diminishes the natural visual range.
Haze is caused by fine particles that scatter and absorb light before it reaches the observer. As the number of fine particles increases, more light is absorbed and scattered, resulting in less clarity, color, and visual range.
Contribution of Various Particulates to Haze
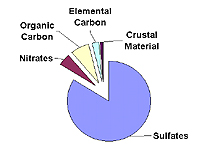
Eastern U.S.
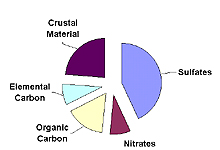
Western U.S.
Five types of fine particles contribute to haze: sulfates, nitrates, organic carbon, elemental carbon, and crustal material. The importance of each type of particle varies across the U.S. and from season to season. The typical importance of each particle type in the eastern and western U.S. is shown in the figure to the right. Details on each particle type are provided below.
Sulfate particles form in the air from sulfur dioxide gas.
Most of this gas is released from coal-burning power plants
and other industrial sources, such as smelters, industrial
boilers, and oil refineries. Sulfates are the largest
contributor to haze in the eastern U.S., due to the region's
large number of coal-fired power plants. In humid environments,
sulfate particles grow rapidly to a size that is very
efficient at scattering light, thereby exacerbating the
problem in the east.
Organic carbon particles are emitted directly into the air
and also form there as a reaction of various gaseous hydrocarbons.
Sources of direct and indirect organic carbon particles
include vehicle exhaust, vehicle refueling, solvent evaporation
(e.g., paints), food cooking, and various commercial and
industrial sources. Gaseous hydrocarbons are also emitted
naturally from trees and from fires, but these sources
have only a small effect on overall visibility.
Nitrate particles form
in the air from nitrogen oxide gas. This gas is released
from virtually all combustion activities, especially those
involving cars, trucks, off-road engines (e.g., construction
equipment, lawn mowers, and boats), power plants, and
other industrial sources. Like sulfates, nitrates scatter
more light in humid environments.
Elemental carbon particles are very similar to soot. They are
smaller than most other particles and tend to absorb rather
than scatter light. The "brown clouds" often seen in winter
over urban areas and in mountain valleys can be largely
attributed to elemental carbon. These particles are emitted
directly into the air from virtually all combustion activities,
but are especially prevalent in diesel exhaust and smoke
from the burning of wood and waste.
Crustal material is very similar to dust. It enters the air
from dirt roads, fields, and other open spaces as a result
of wind, traffic, and other surface activities. Whereas
other types of particles form from the condensation and
growth of microscopic particles and gasses, crustal material
results from the crushing and grinding of larger, earth-born
material. Because it is difficult to reduce this material
to microscopic sizes, crustal material tends to be larger
than other particles and tends to fall from the air sooner,
contributing less to the overall effect of haze.
Haze generally appears either as uniform haze, layered haze, or plumes.
|
A uniform haze degrades visibility evenly across the
horizon and from the ground to a height well
above the highest features of the landscape.
Uniform haze often travels long distances
and covers large geographic areas, in which
case it is called a regional haze.
|

|
|
In a layered haze, you can see the top edge of the pollution
layer. This is often the case when pollution
is trapped near the ground beneath a temperature
inversion.
|

|
|
Plumes result from local sources. Plumes and plume-like
layers of elevated pollution take their shape
under certain meteorological condition where
the air is stable or constrained.
|

|
Some of the pollutants that
form haze have been linked to serious health effects and
environmental damage. Exposure to fine particles in the
air has been linked with increased respiratory illness,
decreased lung function, and premature death. For details, see the health effects page. In addition, sulfate and nitrate particles
contribute to acid rain, which can damage forests, reduce
fish populations, and erode buildings, historical monuments,
and even car paint.
To reduce haze we must reduce
emissions of haze-forming pollutants across broad areas
of the country. Cars, trucks, and industries are much
cleaner than they were in the past, and several programs
are in place to maintain this progress over the next several
years. Nonetheless, these programs by themselves are unlikely
to restore visibility to its natural conditions in many
protected areas.
In April 1999 the U.S. Environmental
Protection Agency (EPA) issued regulations to further
reduce haze and protect visibility across the country.
The EPA and federal land managers from other agencies
are working with state, local and tribal authorities to
promote steady improvements in visibility for decades
to come.
We are challenged to do
our part to help reduce air pollution. To learn more about
what you can do to reduce air pollution, see this EPA webpage.